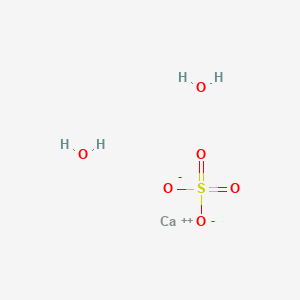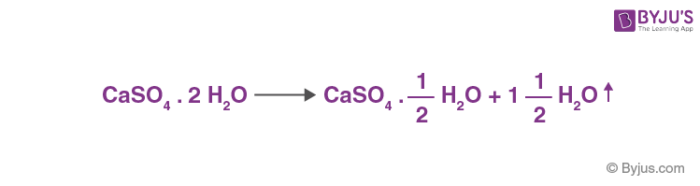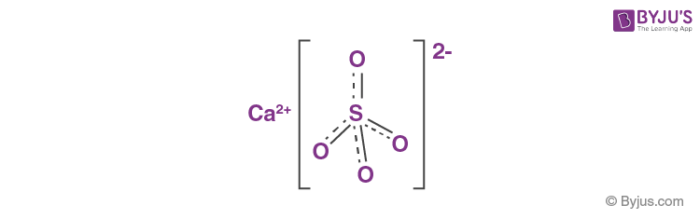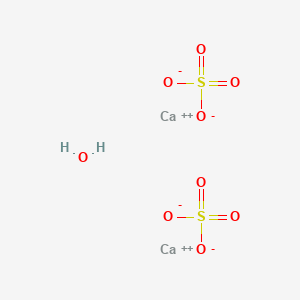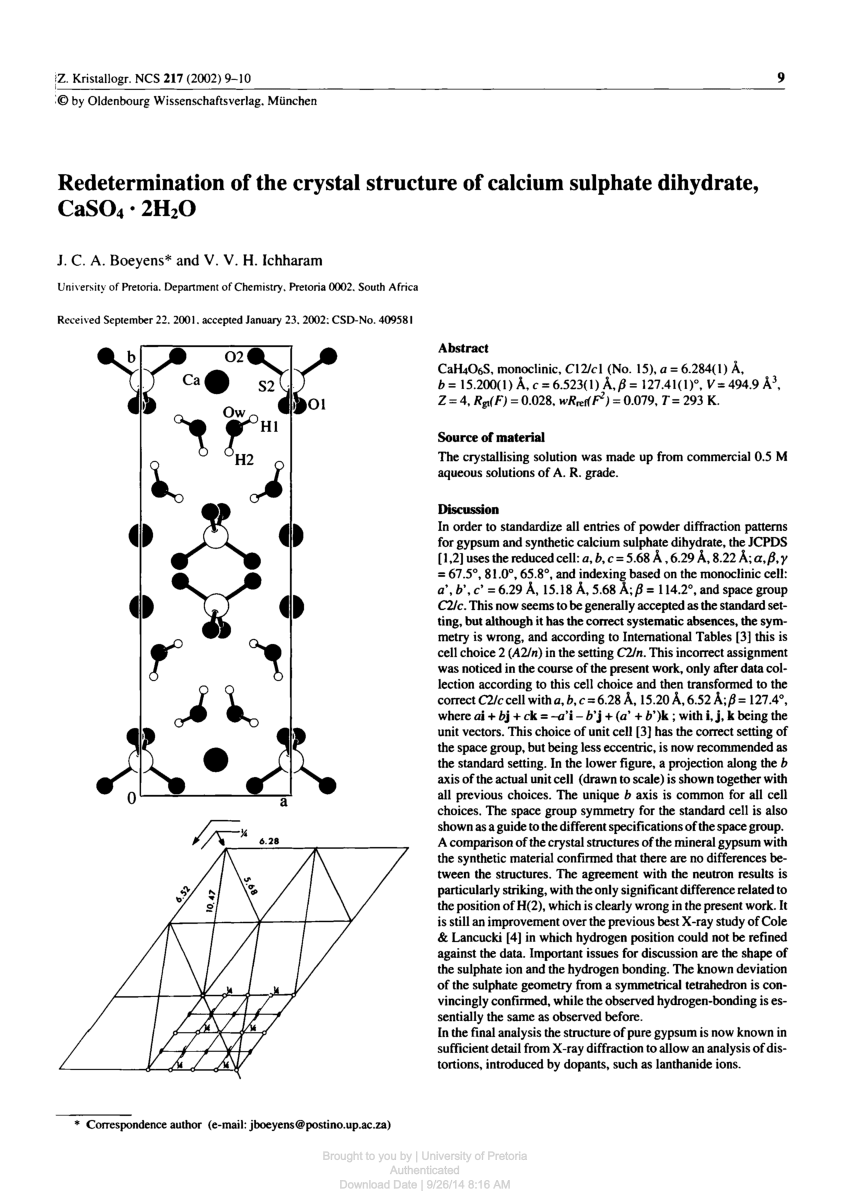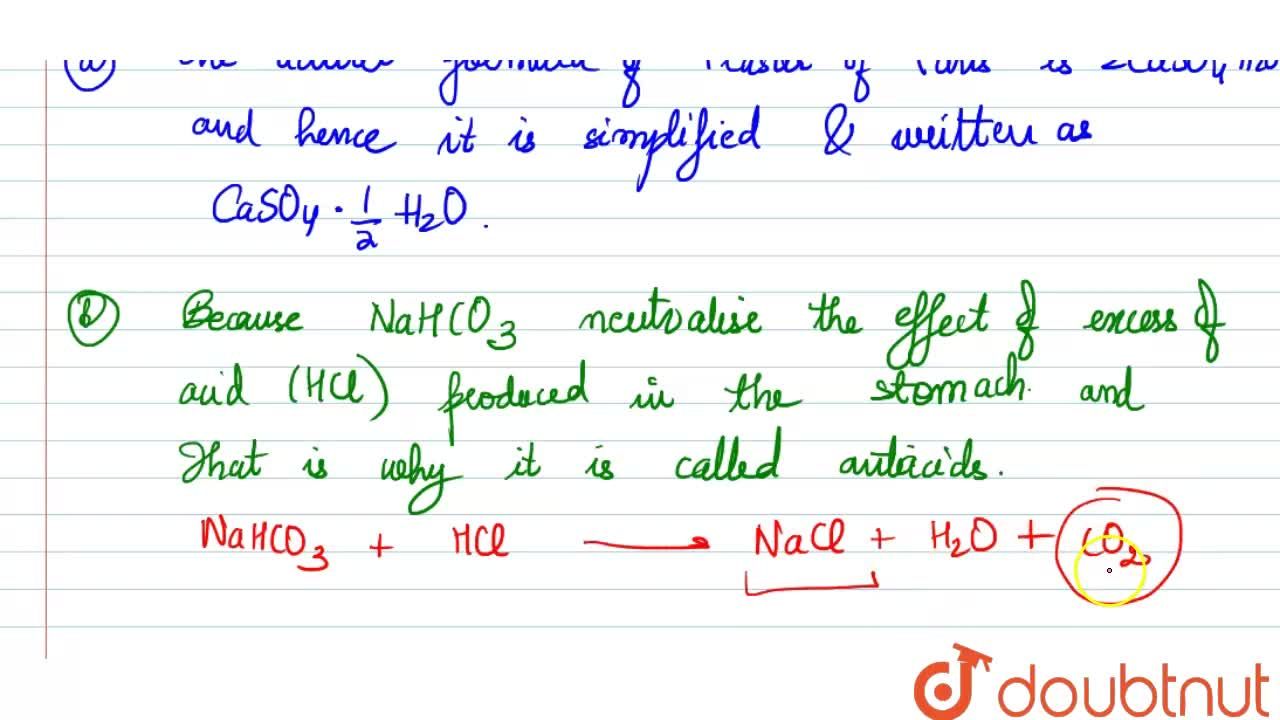
Answer the following : (a) Why is Plaster of Paris written as CaSO(4)1//2H(2)O How is it possible to have half a water molecule attached to CaSO(4) ? (b) Why is sodium hydrogen
![The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1) The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1)](https://dwes9vv9u0550.cloudfront.net/images/5530763/94267030-6cfd-407d-971f-18f86b85f2f7.jpg)
The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1)

Edible Gypsum Powder 10101-41-4 Calcium Sulfate Dihydrate Caso4 2H2O - China Calcium Sulfate Dihydrate Caso4 2H2O and Edible Gypsum Powder 10101-41-4

Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Journal of Chemical & Engineering Data

Reaction Characteristics of CaSO4/CaSO4·1/2H2O Reversible Reaction for Chemical Heat Pump | Semantic Scholar